For as long as I can remember, Humpty Dumpty had a great fall that put him in a tragic state. This rhyme has always been one of my favorites. The story first appeared in 1870, in James William Elliott‘s National Nursery Rhymes and Nursery Songs. Humpty Dumpty is a memorable and versatile teaching lesson, even for adults.
 In my previous post, we looked at what the Three Little Pigs taught me about risk management. Humpty Dumpty also taught me something quite important to pharmacovigilance (PV) and regulatory compliance. (Truly, I’m not stuck in childhood rhymes, but I am amazed at how applicable they are to any stage of life.)
In my previous post, we looked at what the Three Little Pigs taught me about risk management. Humpty Dumpty also taught me something quite important to pharmacovigilance (PV) and regulatory compliance. (Truly, I’m not stuck in childhood rhymes, but I am amazed at how applicable they are to any stage of life.)
The first question that stands out for me is, “Why is he sitting on a wall?” His proportion wasn’t correct for sitting on the wall and fell into the category of “horrible parent-based outcomes.” In today’s regulatory environment, governing pharmacovigilance and drug safety, it’s easy to see how we can become our own Humpty Dumpty.
Choosing a PV or regulatory workflow isn’t stable and allocating our resources disproportionally can be a disaster. What happens when the system fails or becomes overrun? We take Humpty’s fall.
As the rhyme goes, sometimes all the kings’ soldiers and all the kings’ men can’t put your PV/Regulatory system back together again.
So, how do we stabilize in pharmacovigilance and regulatory practices in this world of constant movement and change?
First and foremost, what is your regulatory environment and your geographical footprint? While there are commonalities; not all are a fit. The United States Food and Drug Administration has different centers for different drug categories; EMA and local country affiliates operate differently. Just because one authority has a particular guideline does not mean it will have an iteration from each authority. If you work across regulatory areas, these categories include:
- Human drugs
- Tobacco
- Animal drugs
- Animal vaccines
- Medical devices
- Biologics
- Animal parasiticides
Regulatory affairs is infinitely more complicated. Recently, all the 2013 GxP documents – which was the first move from Volume 9A in EMA in 2013 – were updated. The Middle East, South America and APAC are all evolving at record speed. This should give us the message that the wall isn’t stable.
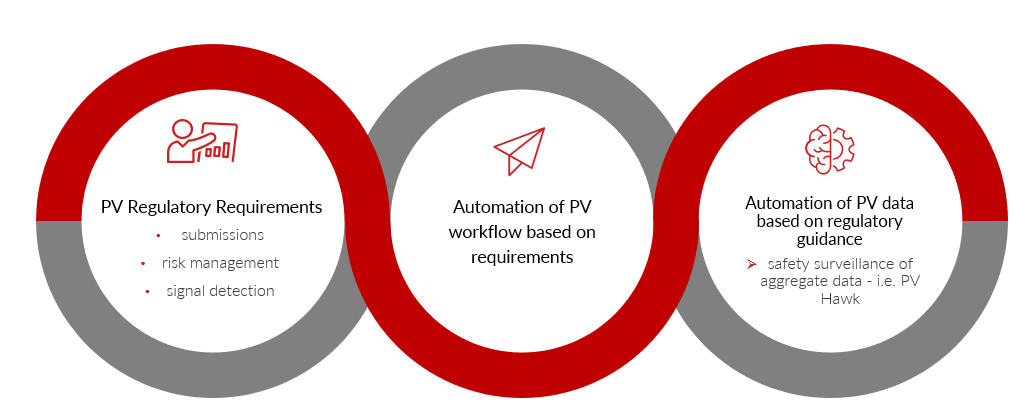
We have moved from a PV system that was based on retrospective analysis to one that expects proactive pharmacovigilance.
This requires faster, more efficient, and more intimate knowledge of our products and the risk benefit paradigm. That also means understanding and complying with the regulations for the full life cycle of our products.
How do you do that without falling off the wall?
None of us at three years of age, while hearing of Humpty’s woes, brilliantly announce to our parents that we are going to be “regulatory affairs or pharmacovigilance” subject matter experts. With the complexity of the new animal health regulations and the updates to the EMA human regulations, regulatory affairs has become more of a specialized regulatory science. That means understanding more, keeping abreast of country based specific regulations, making sure that our PV systems and organizations are compliant, and using the regulations in an efficient manner to develop a regulatory strategy.
We can either ask for an army-sized budget or embrace technology solutions to help. What is available? What is a good fit? What are the end-goals of introducing technology into regulatory affairs and PV?
- Faster and more accurate collection of regulatory information
- Separating relevant vs. non-relevant information
- Approval and distribution of PV regulatory information
- Distribution of information needed for regulatory strategy and design of submission planning
- Ready access to our PV data to rapidly identify potential signals, opportunities for expansion and risk mitigation
The reality for most is that they don’t have enough resources to manage ever-changing regulatory documents – to find, digest, and distribute relevant regulatory information. Each organization wants and needs optimal compliance within the regulatory environment in which they operate. We know that the strategy (both before and after marketing) is unique to the product, indication, geography, and license. The technology should augment and accelerate marketing and compliance. Use technology to automate regulatory documents from global agencies to maximize the use of data, comparative analytics, and automate parts of regulatory submissions.
At Perficient, those technology solutions are our sweet spot. We get it; keeping up with regulations to protect currently marketed assets is difficult and putting together a regulatory strategy to get a drug or device to market is even more complicated. There is also the PV data and analytics.
Technology solutions don’t have to be painful or overly expensive; we believe in fit for purpose.
Check out the diagrams below, contact us for more information (you can also just give us a holler – we all work remotely, so we’ll hear you). Let us show you how to simplify regulatory affairs into a science and survey your PV data based on real time data that is relevant to your unique area. Whether it be animal or human health, we can make it simpler, more accurate, and help you get more with less using technology solutions.
Moreover, your regulatory and PV group will love you for it.

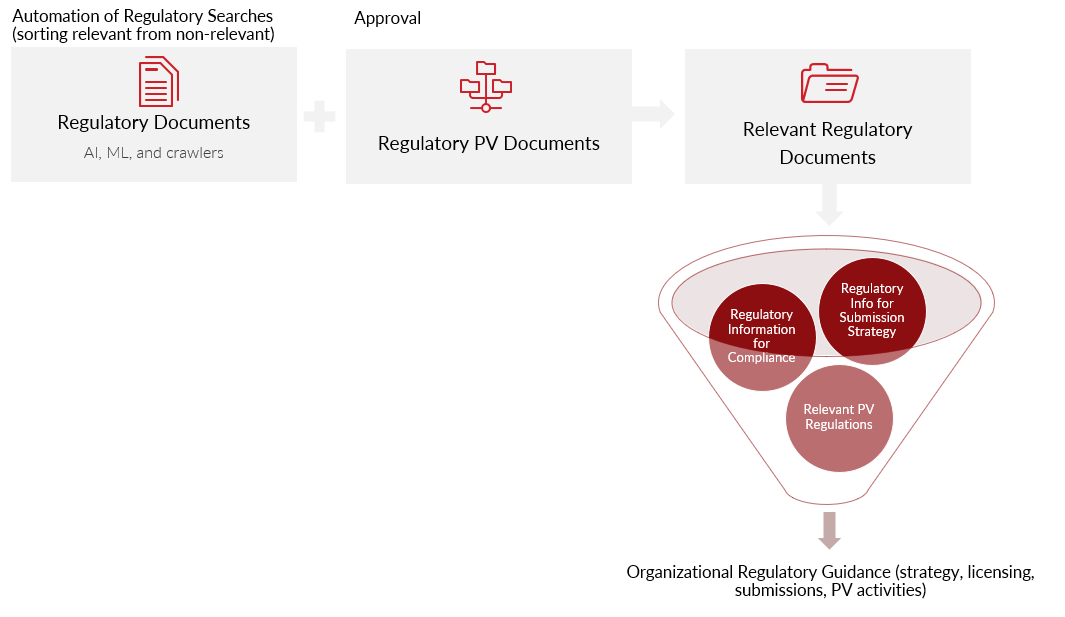
Technology Automation for Surveying Global Regulations and PV Impact:
Kari Blaho-Owens, EMT, Ph.D. is the Director of PV and RA for Healthcare at Perficient. She lives and works in Montana. Kari is a firm believer that finding workable solutions to tough Regulatory, MI, and PV solutions can be found at the end of her fly line. She loves fly fishing, donating her spare time to serve others as a volunteer EMT, and exploring the vast beauty of the state… with the goal of not being eaten by a Grizzly bear.
For PV, MI, call center, and RA conundrums, contact us for workable solutions.

